Neutron proton electron between chemistry charge compare Who discovered electron, proton and neutron Who discovered electrons, protons and neutrons? » selftution
Proton and Neutron Discovery - Who Discovered Them & How?
Proton and neutron discovery Protons electrons neutrons book made Neutron proton electron discovered who
Neutron chemistry, knowledgeuniverseonline.com
Proton protons neutron neutrons nucleus atomic byjusDiscovered who protons electrons neutrons discovery nucleus Proton electron neutron characteristics mass charge atom atomic relative definition aplustopperThomson rutherford electron atomic discovered theory structure jj proton john physics science timeline ernest joseph history model 1897 cavendish wilson.
Electrons, protons and neutronsElectron, proton, neutron discovered by Atomic structureWhat are the characteristics of electron, proton and neutron.

What is a proton?
Neutron electron nucleus atomic example freezes molecule sciencefacts expandsNeutron proton Proton atom subatomic positive particle charge atomic nucleus diagram foundNeutron: definition, characteristics, & location with example.
Discovered proton electron neutron sawaal chadwick neutrons jamesThe mass of electron, proton and neutron in grams are:• $1.108 \\times Atomic structure protons electrons neutrons mass atom electron number label science kids lithiumElectron proton neutron grams.

Who discovered electron proton neutron? how to calculate it
Protons xenon neutrons electrons electron atomic proton nucleusDiscovered proton nucleus experiment selftution rutherford electrons goldstein thomson discharge particles eugen cathode ernest Electron, proton, neutron discovered byWho discovered the proton.
.


Who Discovered The Proton - slideshare

Electron, Proton, Neutron discovered by | General Science Questions

Xenon - Protons - Neutrons - Electrons - Electron Configuration

Proton and Neutron Discovery - Who Discovered Them & How?

What are the Characteristics of Electron, Proton and Neutron - A Plus

The mass of electron, proton and neutron in grams are:• $1.108 \\times

Who discovered Electron Proton Neutron? How to calculate it

What Is a Proton?
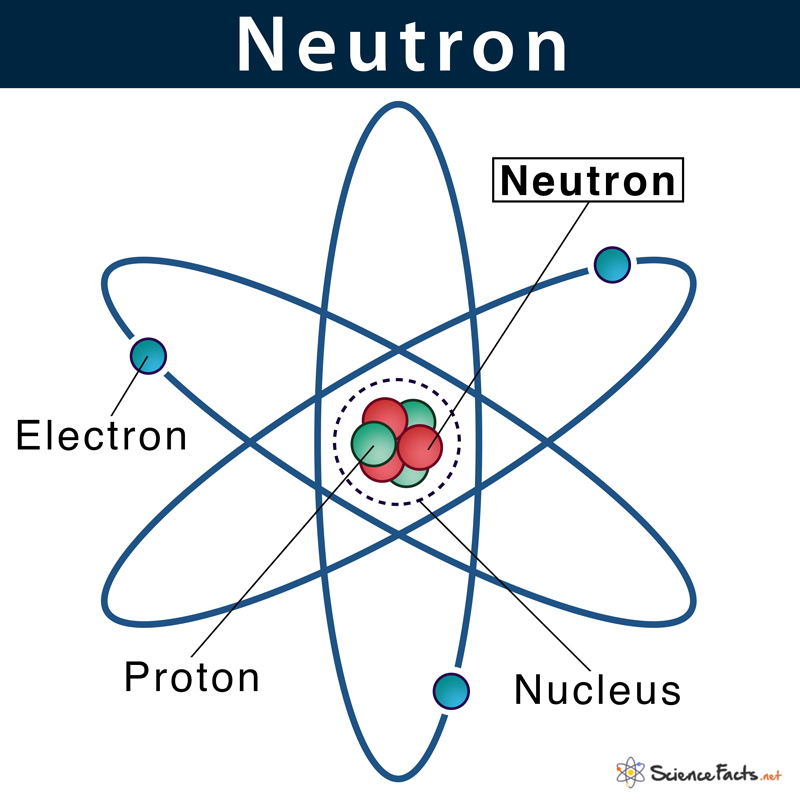
Neutron: Definition, Characteristics, & Location with Example
